Who we are?
SciencePharma is a fast-growing Polish company offering consulting and importing services for pharmaceutical industry. SciencePharma was established in 2004 and now is a leader among Polish and foreign consulting companies. For over a decade we have been successfully implementing national and international projects (EU and non-EU). During this time, we have prepared over 960 dossiers for medicinal products but these are not the only successes on our long list of great achievements.
Our knowledge of the legal requirements, guidelines, procedures and agencies’ expectations in scope and quality of registration dossier, as well as our extensive experience gained through each registration and development project for many pharmaceutical companies is the biggest strength.
We are at your disposal whenever you need support and entrust your issues/ affairs in the experts’ hands. In addition to registration support issues, for several years we have been successfully and holistically handling drug development projects.
We offer support from the early concept stage through the physiochemical development stage, in vitro studies and non-clinical and clinical studies, up to registration and product life cycle (maintenance of authorization, pharmacovigilance etc.). The clinical studies designed by SciencePharma have repeatedly ensured the success of registration procedures and our out of the box approach has resulted in many Rx to OTC switches.
We are able to see different possibilities, suggest alternative action scenarios and above all, we are ready for action even in non-standard situations. We specialize in the importation of medicinal products and investigational medicinal products for clinical trials.
Our experts have wide knowledge and experience in GMP/GDP requirements. Hundreds of audit hours in the manufacturer site and loads of documents studied during QP batch release make us a leader in medicinal products importation and QP batch release.
We have GMP certificate compliance of a manufacturer issued by the Chief Pharmaceutical Inspector. Our interdisciplinary team will eagerly engage even in the most complicated cases, caring for a high quality of our services and customer satisfaction.
Our team’s experts have diversified educational background and scientific achievements (pharmaceutical, medicinal, chemical, biological and law) with extensive experience gained in the pharmaceutical or medical industry, analytical laboratories, the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, the Polish Ministry of Health and so on.


Proud to be part of QbD Group
SciencePharma is proud to be part of QbD Group – a global life sciences partner supporting companies from idea to patient.
By joining forces, we combine SciencePharma’s strong track record in regulatory affairs, CMC development, and GMP/GDP compliance with QbD Group’s broad international expertise across the full product lifecycle. With over 600 experts worldwide – including a growing presence in Poland and China – we offer even more value to our clients through integrated support in regulatory, quality, clinical, lab services, software solutions, and vigilance.
This next chapter allows us to continue doing what we do best – while expanding our impact and capabilities to support clients even better.
Our services
Learn more about the professional consulting services offered by SciencePharma. For 20 years, we have been supporting Polish and foreign clients in obtaining marketing authorization, pharmacovigilance, GxP audits, non-clinical and clinical trials and R&D projects.
We specialize in medicinal products importation thanks to our GMP requirements experts and QPs. We offer all services in the scope of quality of medicinal products, including technology transfer, validation of manufacturing process or GMP system implementation. We offer support in the whole medicinal product life cycle, from development studies to commercialization.
Our wide areas of expertise set us apart from other companies. We have experience not only in medicinal products but also in medical device, ATMP, cannabis, cosmetics and food supplements.

Our achievements
0
R&D projects for medicinal products
0
Registrations dossier (1-5 module) for medicinal products
0
Successfully finished national registrations and European MRP and DCP procedures
0
Medicinal products in our Pharmacovigilance system
0
GMP audits (the EU and third countries)
0
Clinical and observational trials for medicinal products and medical devices
Why us?
We are leaders with 20 years of experience
Worldwide service
Many professionals from different backgrounds
Knowledge of pharmaceutical law
Since we treat our client’s projects as our own, with utmost commitment, we are ready to face all challenges and focus our work culture around client’s needs. Excellent knowledge of pharmaceutical law, experience in cooperation with national registration agencies and a wide range of services are just a few examples of our advantages. Key features that set us apart from other companies:
- Creating optimal and unusual solutions for the clients
- A holistic and interdisciplinary approach to ongoing projects
- Flexibility and fast response to changing requirements
- An extensive, highly qualified team of experts in various fields, open to cooperation
The solutions which we propose are client-tailored. We always set up strategies to be consistent with our client’s vision, but at the same time, we care about meeting all the necessary legal requirements.
A strategy that includes a multifaceted analysis of the project and challenges it entails is, in our opinion, is the key to success. That is the reason why we involve experts from many fields and prepare comprehensive strategies, so from the very beginning of the project we know what awaits us and the client and what challenges we will have to overcome on the way to achieve all our objectives.
Our team is flexible not only in project management and evolving client ideas, but also in constant adaptation to changing requirements and legislation.
We value transparent cooperation and we are open-minded to our clients’ needs. We know that communication is a key to success, so we are glad to have a dialogue with our clients.
Our clients
We have a pleasure to work on pharmaceutical projects for clients from all over the world, that include companies from Poland and other parts of Europe, Asia and Pacific Region, Africa and North and South America. For years, we have been supporting the pharmaceutical industry, as well as representatives of the science world. We cooperate with companies that have generic products portfolio as well as with the ones that have innovative products, including biotech companies. We cater our services to companies of any size, from small and innovative start-ups to medium and large corporations. No job is too small or too big for us. We have been cooperating with manufacturers of vaccines, blood products and API producers. Our clients include manufacturers of medical devices, veterinary products, cosmetics and food supplements. We worked with universities, science institutes and other research units.
- Europe
- Africa
- North America
- Asia & Pacific
- South America
- Canada
News & insights
10 Dec 2025
Read more
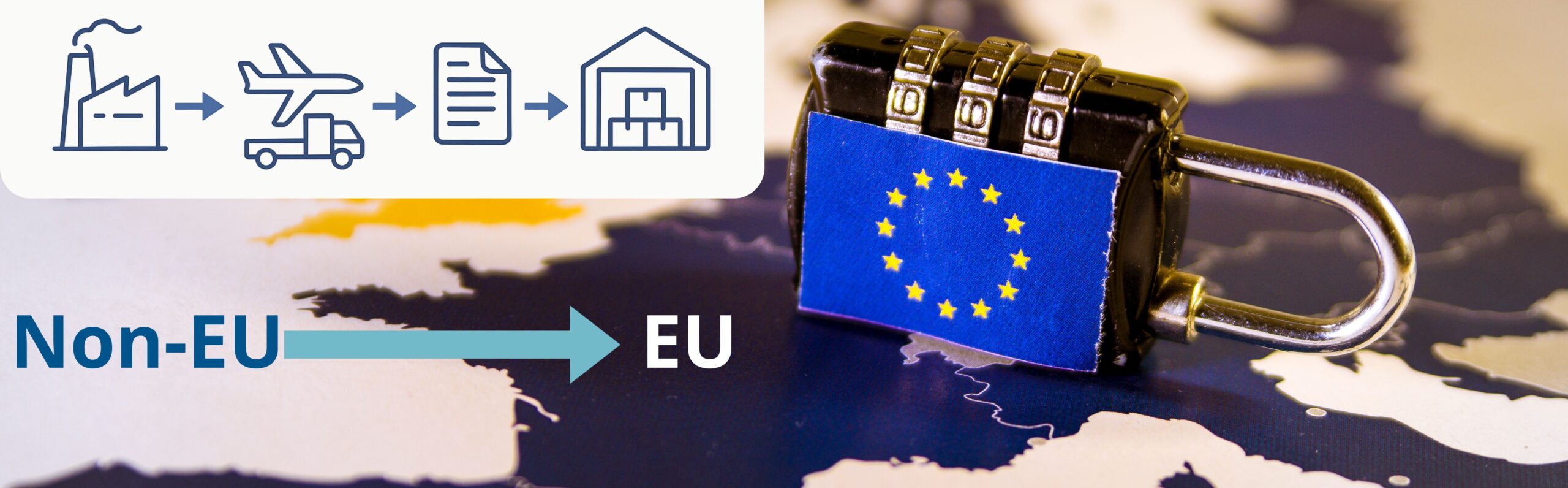
Importing Medicinal Products: GMP Requirements and Customs Clearance Challenges
Importing medicinal products into the EU is a highly regulated process that leaves no room for error. From strict GMP expectations to complex customs procedures, each step can affect timelines and product quality. This article explores the main regulatory challenges and highlights practical strategies that help importers avoid delays, ensure compliance, and protect product integrity. Key GMP Requirements for […]

Importer’s Warehouse – Permits, GMP Requirements, and Key Differences from a Pharmaceutical Wholesaler
Read more
How can an internal Quality Management System support a company that operates in accordance with regulations?
Read more
Safety data exchange: modern must-have to maintain Pharmacovigilance
Read more