
GMP: Support in preparation to inspection
Preparing the manufacturing site to achieve the goal of GMP certification and obtaining a permit for manufacturing/ importation authorization is an undertaking that involves the entire team. Knowledge of the product and its manufacturing process, as well as legal regulations relating not only to GMP, but also to registration issues is crucial.
The manufacturing/import authorization (MIA) is the most important document to obtain to start manufacturing activities in the EU for medicinal products (including investigational medicinal products). It enables manufacturers to start manufacturing activities. The GMP Certificate confirms compliance of manufacturing conditions with GMP requirements. It is mandatory for manufacturers from third countries to start manufacturing activities, while for manufacturers based in the EU, it is an additional document supplementing the manufacturing permit.
For young manufacturers that will be subject to a GMP inspection for the first time, preparing for the inspection is a considerable challenge, during which the multitude of questions that appear at every step can be overwhelming.

- How to request an inspection?
- Which GMP regulations apply to the factory?
- Which qualifications should be completed during the inspection, and which may be in progress?
- Is it necessary to perform a full validation process and analytical methods for the products covered by the inspection request?
- Finally, what is the difference between a manufacturing permit and a GMP certificate?
It is not only young manufacturers that may need help interpreting legal provisions. Manufacturers operating in the pharmaceutical market for years face the challenges posed by constantly changing GMP regulations. Only in recent years a significant change in GMP regulations has been implemented regarding the implementation of the long-awaited GMP Annex 1, which became effective in European regulations on March 25, 2023 (for point 8.123 from April 25, 2024). GMP Annex also causes some challenges to production plants that have been on the EU market for many years. An example might be point 8.123 regarding requirements for freeze dryers, which for some plants may involve extensive renovation and construction works, or the requirement to introduce a Contamination Control Strategy (CCS).
These and many other questions will arise as the plant’s quality system is prepared for GMP inspection. During the demanding preparations for the inspection, it is worth considering support from Consultants who will provide support during this intensive period of preparation for the inspection, supporting and relieving the plant staff. An important question before the inspection is whether my GMP quality management system works appropriately or contains any gaps. In such cases, Consultants perform an audit of the system, finding any deficiencies that require correction, and will pay attention to critical aspects. Then, if necessary, they will take corrective and preventive actions or train the personnel accordingly. Consultants also provide support during and after the inspection, actively participating in preparing responses to the inspection report and implementing post-inspection recommendations.
The appearance of these documents generates many difficulties and questions for manufacturers. In the case of Annex 1, the difficulty may be that this still needs to be implemented into Polish law. Does this mean this document is currently not interesting to Polish manufacturers? It is because work on the translation and implementation to Polish law is ongoing, but it is still not known when the document will be implemented.
Why SciencePharma?
The strength of SciencePharma is that we are a multidisciplinary team of experts with extensive knowledge in the field of GMP, both in terms of regulatory and practical knowledge. Among us are pharmacists, chemists, technologists, biologists, and biotechnologists. Our experts have experience working in pharmaceutical factories, research institutes, and universities, as well as in offices such as the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products (URPL). The extensive knowledge of our experts allows us to look at each problem from different perspectives, taking into account the impact of the decisions made on the future activities of the production plant. Our Experts have extensive experience in the field of GMP for marketed and investigational medicinal products, both for sterile and non-sterile products manufactured in various pharmaceutical forms. In addition, we have many years of experience in supporting our clients in preparations for GMP inspections and in post-inspection activities, including contacts with, among others, the Main Pharmaceutical Inspectorate (GIF) and the European Medicines Agency (EMA).
Benefits of cooperation
We provide comprehensive customer support during preparations for the inspection and post-inspection activities.
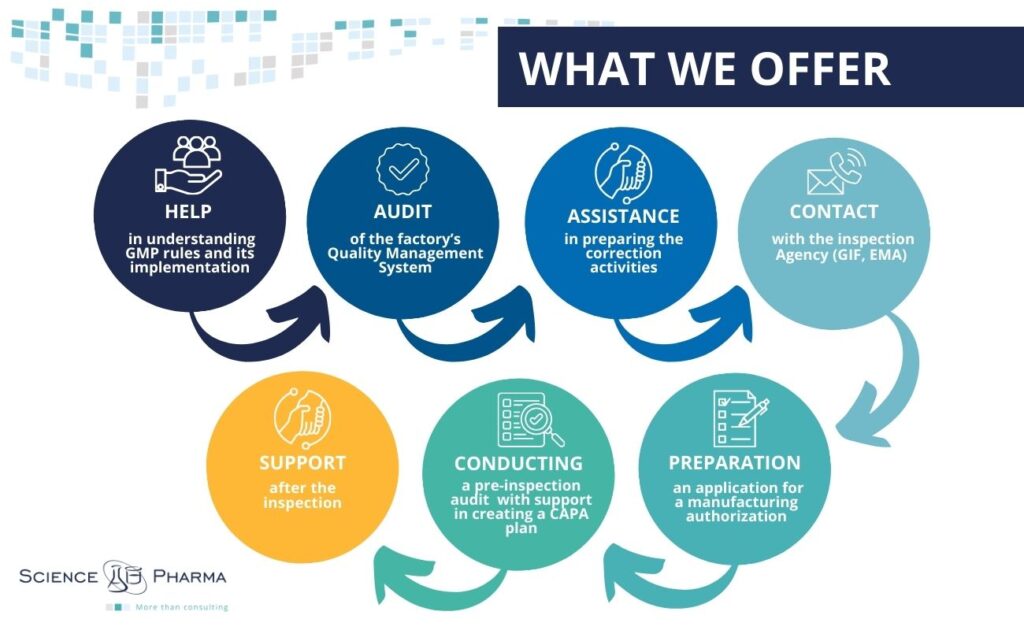
We offer our assictance during following actions:
- support in understanding GMP rules and its implementation,
- audit of the factory’s Quality Management System for compliance with GMP regulations,
- assistance in preparing the correction activities,
- contact with the inspection Agency (GIF, EMA),
- preparation of a strategy for applying for a manufacturing authorization,
- preparation of an application for a manufacturing authorization,
- conducting a pre-inspection audit with support in creating a corrective action schedule (CAPA plan),
- support during the preparation of CAPA plan after the inspection.
The scope of our support is tailored to the individual needs of our Clients. Whether you need to implement the EU GMP requirements or others (US GMP or WHO GMP), please contact us so we can choose the most suitable solution for you. Find out more about GMP audits.

