
Own importer warehouse – safe storage and comprehensive pharmaceutical import services
At SciencePharma, our modern warehouse is a key element of our import services. As a professional importer of pharmaceutical products, we offer comprehensive support for bringing medicinal products manufactured outside the EU into the European Union, ensuring proper storage and distribution.
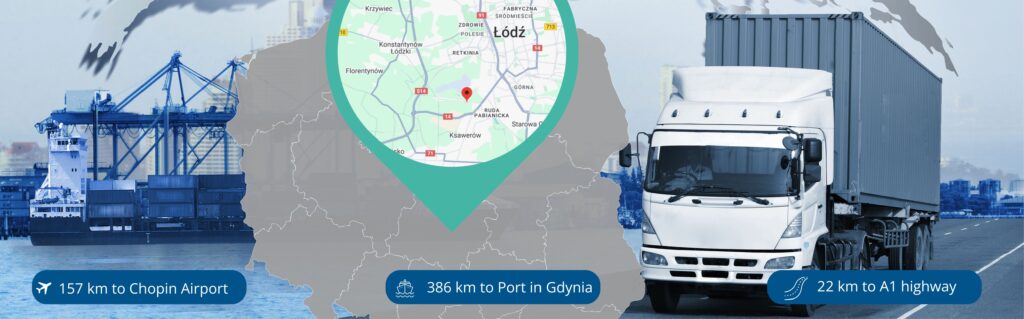
Warehouse location in the heart of Poland – an essential element of logistics operations
Our importer warehouse is located in the southern part of Łódź, directly by National Road No. 14. It has convenient access to the S14 expressway and the A1 and A2 highways, making it ideal for companies looking to efficiently distribute medicinal products not only in Poland but also across the European Union and beyond.
An additional advantage of the location is the proximity to major seaports in Poland – Gdańsk and Gdynia, which allows for easy and fast sea transport of medicines. This makes our offer flexible and thoroughly adapted to the needs of international pharmaceutical markets.
Modern storage systems and efficient space management in our warehouse
Its state-of-the-art facility ensures an advanced storage system and the highest safety and quality standards for pharmaceutical product storage. All warehouse rooms have advanced temperature and humidity monitoring systems, regularly validated to guarantee optimal storage conditions. Our warehouse complies with all applicable norms and regulations for storing medicinal products, ensuring compliance with industry requirements.






Comprehensive Pharmaceutical Import Services
SciencePharma offers warehousing and complete pharmaceutical import services. Our MIA (Manufacturing and Importation Authorization) license covers a wide range of services, including importing and certifying medicinal products from outside the European Union (both investigational and market products) and storing medicines for clinical trials, registered products, and controlled substances.
As a company committed to the highest quality standards, our Quality Management System (QMS) complies with GMP (Good Manufacturing Practice) requirements, ensuring that all stored pharmaceutical products meet strict safety and quality standards.
Why choose SciencePharma?
- Own Importer Warehouse – we have a modern warehouse in central Poland, ensuring fast access to pharmaceutical products.
- Compliance with GMP – our Quality Management System meets GMP standards, guaranteeing the highest quality of stored medicines.
- Comprehensive Import Services – we offer full support for importing, certifying, storing, and distributing medicinal products, including clinical trial medicines and controlled substances.
- Wide Range of Services – with our MIA license, we ensure full compliance with EU regulations, enabling the import of medicines from non-EU countries into the EU market.
Trust the Experts in Pharmaceutical Import
By choosing SciencePharma as your partner, you can be confident that the import and distribution process will comply with applicable regulations and that your products will be stored safely and correctly. Rely on our expertise, experience, and innovative solutions that support the growth of your business.
Contact us to learn how we can support your pharmaceutical import activities.

