Have any of you used them? Had a contact with them? Nanoparticles are everywhere around us. Undetectable by the human eye, nanoparticles can exhibit significantly different physical and chemical properties to their larger counterparts. Researchers realized the importance of nanoparticles when they found that size can significantly influence the physiochemical properties of such substances.
Although, currently, there is a coexistence of different definitions of nanomaterials in the EU which causes discrepancies between some sectors, according to the European Commission recommendation issued in 2022, nanomaterials are natural, incidental, or manufactured material consisting of solid particles present, either on their own or as identifiable constituent particles in aggregates or agglomerates where 50% or more of these particles in the number-based size distribution fulfils at least one of the following conditions:
- one or more external dimensions of the particle are in the size range 1 nm to 100 nm,
- the particle has an elongated shape, such as a rod, fibre, or tube, where two external dimensions are smaller than 1 nm, and the other dimension is larger than 100 nm,
- the particle has a plate-like shape, with one external dimension smaller than 1 nm and the other dimensions larger than 100 nm.
To imagine nanosize, remember that the diameter of a human hair is 100 µm, and a human cell is 10 µm. Bacteria are ~1 µm in size, while water molecule is ~0.1nm
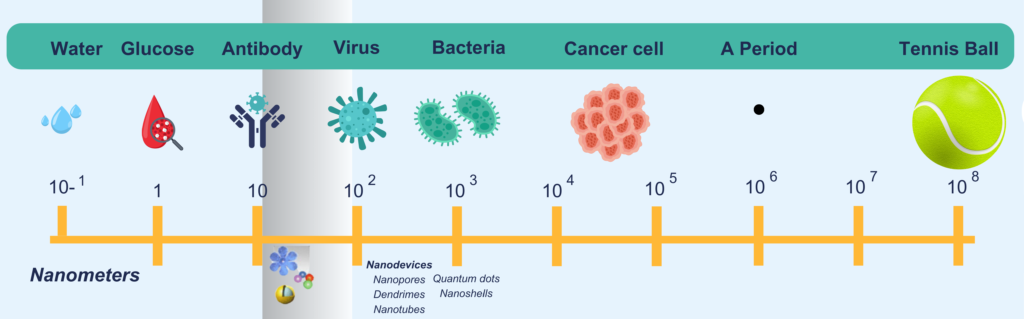
Fig. 1. The size of nanoparticles in relation to other objects
Nanotechnology is helping to improve, even revolutionize, many technology and industry sectors, including information technology, homeland security, cosmetics, transportation, energy, food safety, environmental science, and medicine. The sector with the greatest potential for expansion and development over the next decade is nanomedicine. The words nanomedicine, nanotechnology, and nanodevices are used in the public sphere, not just in the scientific one.
Nanoparticles presence around us
Let’s take a closer look at nanoparticles and their presence around us. Now, we can probably say that nanoparticles are everywhere, and we have daily contact with them. Do you believe 5 nm nanoparticles were found in the most popular worldwide beverages? Note that some nanoparticles occur naturally in some foods; a good example is milk. Casein micelles in milk are protein nano-spheres, which are more available for us to absorb. Ubiquitous additives in food are titanium dioxide, a whitening agent, or silicon dioxide, an anticaking agent; both usually contain microsize particles and nanoparticles. Nanoparticles can quickly and easily reach taste buds on the tongue, so their level of use could be decreased. Nanoparticles might also extend food shelf life or reduce the need for added fats.
Nanomaterials not only impart critical advantages but can also cause toxicity because of their unwanted interactions with biological and cellular processes. The possible passage of nanomaterials into air, water, and soil ecosystems could result in diverse environmental impacts. Every day, in the public domain, we can read or hear about nanoplastic pollution, the detection of nanoplastics in wild organisms, and some aspects of the open debate on nanomaterial safety.
Many of us probably asked whether we have anything to fear. No, because the European Food Safety Authority (EFSA) ensures food safety in Europe. Recently, EFSA published a new approach to risk assessment, including a discussion of challenges on assessing the safety of nanoparticles in food [1]. EFSA recommends a detailed physicochemical characterization of nanomaterials, covering toxicodynamics and toxicokinetics. According to all EFSA regulations and recommendations, the focus during the assessment has to be on:
- degradation/dissolution,
- genotoxicity,
- cytotoxicity,
- oxidative stress,
- and (pro-)inflammation characteristics.
To summarize, there is no need to worry. EFSA monitors and regulates the requirements for food and feed products containing small particles, including nanoparticles.
Medical applications of nanoparticles – Nanotechnology Product Database and scientific guidelines
Let’s move on to the medical applications of nanoparticles. The Nanotechnology Product Database currently (March 2025) contains 1318 products from 578 companies and 48 countries. Interestingly, the database divides a product into six categories:
- dentistry (90 products),
- disinfection (103 products),
- medical supplies (506 products),
- pharmaceutics (432 products),
- prosthesis and orthopedy (26 products),
- and tissue engineering 61 (products) [2].
The European Medicines Agency (EMA) published scientific guidelines on nanomedicines, which help drug product developers prepare marketing authorisation applications for nanohuman medicines. A complete list of EMA scientific guidelines regarding nanomedicines can be found here.
Moreover, for Marketing Authorization Applications (MAA) in Europe, the regulatory system allows regulators to provide “scientific counselling” to applicants from the early stages of research and development.
Advancements in nanotechnology for drug delivery
Every material’s properties change as its size approaches the atomic scale due to the increasing surface area to volume ratio. Nanoparticles’ very small size and high surface area to volume ratio, compared to non-nano-size materials, are the unique features causing nanoparticles’ extraordinary optical, physical, and chemical properties. It’s worth noting that nanoparticles are small enough to confine their electrons and produce quantum effects.
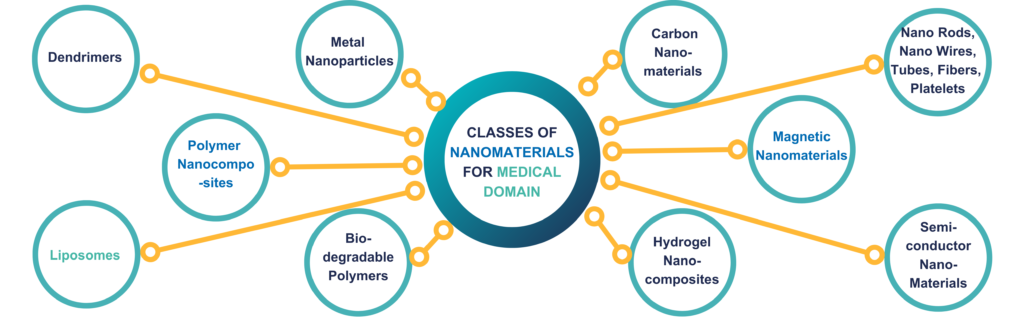
Fig. 2. Various classes of nanotechnology-based materials for medical application [3]
Nanoparticle production methodologies
Advanced single-step and multi-step nanoparticle production and isolation methodologies are being developed and used:
- RESS (Rapid Expansion of Supercritical Solutions),
- SAS (Supercritical antisolvent),
- SAS-WTS (Supercritical antisolvent process integrated with Wurster type coater),
- SAS-EM (Supercritical Antisolvent Precipitation with Enhanced Mass transfer),
- SpEDS (Suspension-Enhanced Dispersion by Supercritical Fluid),
- SAS-DEM (Supercritical antisolvent – Drug Excipient Mixing),
- RESS-WTS (Rapid Expansion of Supercritical Solutions process in combination with the Wurster coater),
- RESS-BFB (Rapid Expansion of Supercritical Solutions process in combination with the Fluidised Bed),
- SAS-FB (Supercritical Antisolvent Precipitation with Fluidised Bed).

Fig. 3. Single-step and multi-step nanoparticle production and isolation methodologies [7]
Nanoparticles advancing the development of poorly water-soluble API’s medicinal products
Nanoparticles also play a crucial role in solving one of the pharmaceutical industry’s huge challenges: the poor solubility of many active substances in water. Nearly 40-50% of the valuable oral drugs marketed in the USA and Europe and almost 90% of the new chemical active substances in the drug development stage are poorly water-soluble. For poorly soluble APIs, nano-drug delivery systems and techniques open a new era for generating nanoproducts, which includes, e.g.:
- canonization,
- complex or micelle formation,
- encapsulation,
- nanosuspension formation,
- supercritical fluid,
- high gravity precipitation,
- no precipitation techniques,
- nano gels, or nano matrices formation.
Old, well-recognized techniques, like spray drying and freeze drying, are also used in the new version.
Nano-based systems are also attractive for many other reasons, including their ability to protect APIs from degradation, both in storage and after the drug is administered to the patient, during the API’s transport to the target site within the body. Moreover, nanoparticles can affect the drug interactions with the cells at the target site. Finally, nanoparticles can influence the distribution and time of product circulation before elimination.
Scope of currently approved medicinal products containing nanoparticles
Over the last two decades, the Food and Drug Administration (FDA) and EMA have approved nearly 80 nanoparticle products [4]. Currently, the majority of approved therapeutic nanoparticles are in the EU market. The available nano-drugs demonstrated that they:
- enhance active substance bioavailability,
- eliminate some side effects,
- and deeply increase the therapeutic effect overall.
The first nano-drug for cancer treatment was a PEGylated liposomal formulation of doxorubicin. Nowadays, many nanocrystal-based and lipid-based nanoparticle products are available on the market. One of the last top uses is lipid nanoparticles as a delivery system for nucleic acids, including mRNAs in the COVID-19 vaccine [5,6].
Many drugs are in the pipeline, such as products based on:
- soy phosphatidylcholine (SPC-3),
- cholesterol,
- dipalmitoyl phosphatidyl glycerol (DPPG),
- methoxy-PEG-stearoyl phosphatidyl ethanolamine (mPEG2000-DSPE),
- chitosan,
- number of modified polymers,
- silica-based nanoparticles,
- metallic nanoparticles,
- carbon nanotubes,
- dendrimers
- or nanodiamonds.
Nanoparticles in regenerative medicine
A challenging interest of nanoparticles in medicine is regenerative therapy, which mainly focuses on designing new biocompatible materials to enhance tissue repair and regeneration. As life expectancy increases, interest in developing and directly administering therapeutic nanoparticles to promote bone regeneration increases. The most advanced nano-delivery systems for bone regeneration are:
- synthetic PLA or PLGA,
- or natural polymers such as collagen, gelatin, albumin, and chitosan.
Besides polymeric, various formulations of non-polymeric nanoparticles (silica-based, metallic) have also been used as nano-delivery systems for bone regeneration. Calcium phosphate-based non-polymeric nanoparticles are mostly used due to their similarities to human bone.
Micro/nanomotors for regenerative medicine are developing rapidly. Main design strategies include:
- 3D-printing,
- microfluidic,
- biohybrid,
- and polymer template methods [8].
Soon, what is probably not easy to imagine for all of you is that nanorobots can be one of the options for cancer treatments; many such vehicles have made a way from theory to practice, from in vitro experiments to in vivo applications [9]. A crucial aspect for EMA to register such a product will be explicit confirmation of the mechanisms of interactions between nanorobots and proteins/cells/tissues/organs and the proper and broad nanomaterials characterization with particular attention on biosafety and clear in vivo metabolic behaviour.

Challenges and future potential of nanoparticles in medicine
Nano-based technology has made enormous progress over the last decades. Currently, there are huge numbers of products, not only medicines, that either contain or require nanoparticles for their manufacturing and functionality.
Nanotechnology offers many advantages in various fields of science and life. Many drug products on the market have proven the potential of nanoparticles in many medical applications. Nanoparticles overcome some challenges associated with conventional therapy. However, some issues like side effects and toxicity are still discussed and unsolved. Despite their undoubted limitations, a high level of understanding, an advanced level of development, and a huge range of different biological interaction testing will definitely achieve many strategies for treating, preventing, and diagnosing many diseases, particularly those still untreatable.
SciencePharma experts offer support in designing development studies, pre-clinical and clinical trials, as well as preparing complete dossiers. We assist in innovative projects that have the potential to shape the future of medicine.
References
- https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2024.EN-8826
- https://product.statnano.com/industry/medicine
- Abid Haleem, Mohd Javaid, Ravi Pratap Singh, Shanay Rab, Rajiv Suman, Applications of nanotechnology in medical field: a brief review, Global Health Journal,7, 2,2023, https://doi.org/10.1016/j.glohj.2023.02.008. https://www.sciencedirect.com/science/article/pii/S2414644723000337
- https://pmc.ncbi.nlm.nih.gov/articles/PMC8777701/
- https://www.sciencedirect.com/science/article/pii/S1773224722004646,
- https://pubs.rsc.org/en/content/articlepdf/2024/nr/d4nr00019f
- Vivek Verma, Kevin M. Ryan, Luis Padrela, Production and isolation of pharmaceutical drug nanoparticles, International Journal of Pharmaceutics,603,2021,https://doi.org/10.1016/j.ijpharm.2021.120708. https://www.sciencedirect.com/science/article/pii/S0378517321005135
- https://www.sciencedirect.com/science/article/pii/S2590049822000777
- Kong, X., Gao, P., Wang, J. et al. Advances of medical nanorobots for future cancer treatments. J Hematol Oncol 16, 74 (2023). https://doi.org/10.1186/s13045-023-01463-z