Since the prehistoric times people have used medications, primary in form of medicinal plants. Palaeolithic humans built on and expanded their knowledge on active substances present in herbal plants [1]. Just as knowledge about possible treatments has evolved over time, so has increased the awareness of drug safety and possible adverse drug reactions that might occur. Patients should be broadly educated how to use drugs in a safe and efficient manner, how to store medicines so that they do not change their beneficial properties and if any adverse reaction occurs why and where to report it. In this article we would like to highlight these subjects to grow the awareness of proper usage of medicinal products.
Safe use of medications
Each administration of medicinal product is associated the risk of undesired effects that might occur in the course of the treatment. These are called adverse drug reactions (ADRs). According to the definition provided by the European Legislation, adverse drug reaction is: ‘a response to a medicinal product which is noxious and unintended’. This definition in its broad meaning describes a comprehensive range of undesired situations which may arise after drug administration [2]. Involuntarily the question arises, how to prevent such noxious response? Each treatment with a drug should be preceded with thorough familiarisation with the patient information leaflet. This document placed inside each outer packaging of the drug product is a mine of knowledge about the medicine. Getting to know with all the details regarding proper administration of the medication – route of administration, posology, possible side effects, contraindications as well as possible interactions with other drugs and what is equally essential interactions with food is crucial. [3]
Besides that, the patient or care giver should always bear in mind some golden rules of using medicines. These are called “the five rights” and were mainly developed for nurses, but in fact apply to home treatment as well. These rights include:
- Right patient – drugs are meant to be used by a specified patient for whom they were prescribed. When used in a person who does not suffer from the indicated illness, they may cause more harm than good.
- Right drug – it is of high importance to ensure that the medication to be administered is the right product. Many products may look similar, but might contain active substance which is essentially different from the one meant for the particular patient.
- Right route – medications can have many different routes of administration, all of which vary in time to absorb, time to act and potential side effects.
- Right time – each drug should be used in regular time intervals as it was specified by the physician or described in the leaflet. Some products are meant to be taken only in the morning, before, with or after meal.
- Right dose – incorrect dosage may cause extremely serious side effects. Where low dose may result in lack of effect of the product, over dose may give rise to drug poisoning [4].
What if the adverse drug reaction occurs?
However, even when used properly, administration of medicinal is always is at risk of occurrence of adverse drug reactions (ADRs). Some might be surprised to learn that reporting ADRs isn’t limited to healthcare professionals and that EVERY adverse side reaction should be reported. Why is it so important? Collection of all side effects, no matter whether expected or not, either serious or not, allows to gain comprehensive knowledge regarding the drug safety.
After filing ADR the report is added to a national database of drug side effects, which is monitored for patterns. Healthcare regulators analyse the data to determine the frequency and severity of reported ADRs. If necessary, more in-depth research is conducted to evaluate the risks associated with the drug. Depending on the findings, proper actions are taken which may include updating drug labelling with new warnings, issuing safety alerts, dose adjustments, or even removal of the drug from the market.
The results of these investigations gives also a feedback into the healthcare system, informing future prescriber practices and patient education. That’s why it is so important for the common improvements in medication safety and help in better understanding a drug’s risk profile.
What about the extremely rare and hard-to-predict adverse drug reactions? Do they matter? Of course they do, and reporting them is out of extreme importance. In line with the Nassim Nicholas Taleb theory, such extremely rare and hard-to-predict adverse drug reactions are called black swans. [5] These reactions may not occur during the clinical trials due to limited size of the study population. That is why it is so important to report each adverse reaction that follows drug administration.
Who Can Report ADRs? What Should Be Included in a Report?
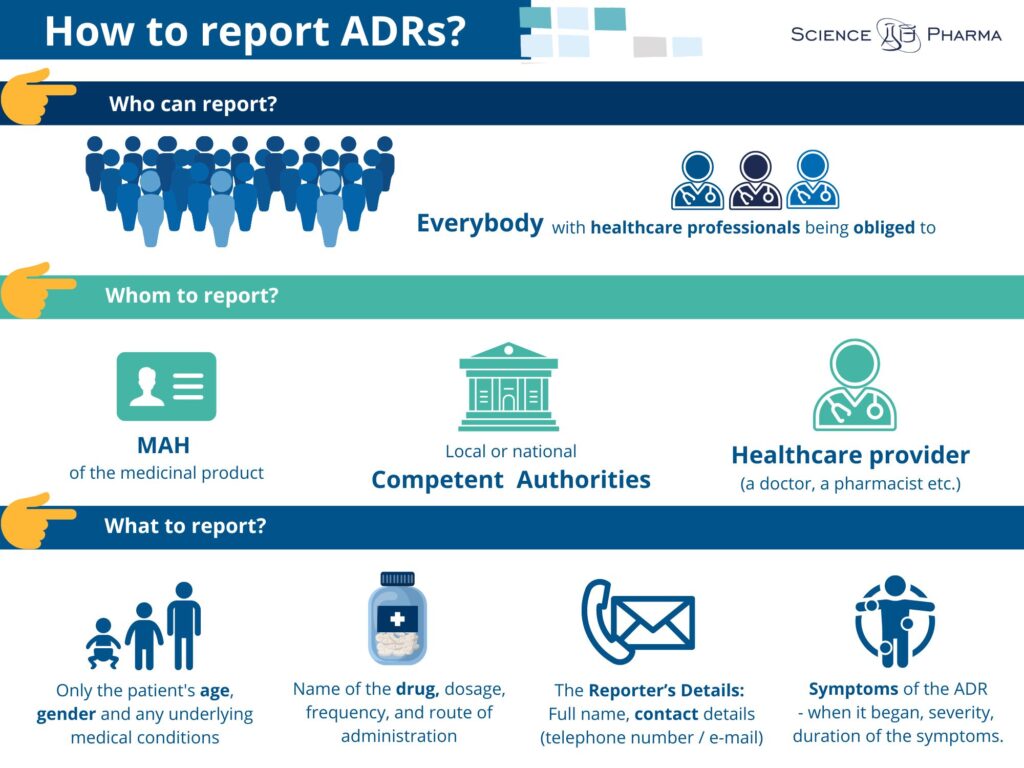
Who can report the side effects? In compliance to the effective regulations everybody is allowed to report side effects. In fact, there are even some groups of healthcare professionals who are legally obliged to do so. ADRs may be reported by anyone (not only by healthcare professionals), but also by each patient himself, caregiver or any member of the general public. When reporting ADR it is crucial to include essential information including:
- The Patient’s Details: Age, sex, and any underlying health conditions (no need for personal identifiers like name or address for privacy reasons).
- The Reporter’s Details: Full name, contact details (telephone number or email)and when The Reporter is a Healthcare Professional – his/her workplace address.
- The Medicinal Product Information: Name of the drug, dosage, frequency, and route of administration.
- Symptoms of the ADR: Detailed description of the reaction, including when it began, its severity, and the duration of the symptoms.
What’s more when reporting ADR other valuable information (if known) should be contained, such as:
- Any Concurrent Medications: Other drugs or supplements being taken, which might interact and contribute to the ADR.
- Outcome: Specify whether the symptoms were alleviated after stopping the drug or reducing the dose.
Where to report ADRs?

Patients are highly encouraged to report to healthcare professionals. This way the report shall be medically confirmed. However it is important to know there are several channels for reporting ADRs. You can:
- Report directly to the Marketing Authorisation Holder (MAH). The contact details are nowadays available on the leaflet of the particular product as well as can be easily found online.
- Report to local or national drug regulatory authorities
- Inform your healthcare provider, who can report on your behalf.
How to report? Both MAHs and Agencies have developed dedicated adverse drug reaction forms (AERFs) for reporting of side effects and made them available to download from their web pages [6].
Proper storage of drug products
Last but not least, for the safety of medicinal products use be sure you are keeping it an appropriate conditions. Storage conditions of each product are described in the patient information leaflet. Those conditions are based on the results of stability testing performed by the MAH. Each factor of the environment may influence the properties of the medication causing worsening of its quality together with posing a threat to patient’s safety.
What should the patient pay attention to:
- Temperature
- Humidity
- Exposure to sunlight
What’s more all medicinal products should be stored in original packaging which is designed to protect the product from the environmental impact including the contamination of the drug. Do not leave your medicines in a hot car. Do not buy medicines from store windows where they are left in the sun. Be careful with buying drugs online, especially when you are not sure about legality and relevance of the source. Remember, all medicines should be kept away from children. Children as a population of extremely curious and experimenting human beings may accidentally be exposed to drug products which may jeopardise their health. [7,8]
In conclusion, medicines are present with us since the prehistoric times. Human beings through the ages have broadened their knowledge regarding efficacy and safety of numerous active substances. Thanks to this, keeping in mind all the above mentioned rules, each patient can reap the benefits out of therapy avoiding threatening their health.
Sources:
- Hardy K. Paleomedicine and the Evolutionary Context of Medicinal Plant Use. Rev Bras Farmacogn. 2021;31(1):1-15. doi: 10.1007/s43450-020-00107-4. Epub 2020 Oct 9. PMID: 33071384; PMCID: PMC7546135.
- Directive 2001/83/EC.
- Pines A. Patient information leaflets: friend or foe? Climacteric. 2015 Oct;18(5):663-5. doi: 10.3109/13697137.2015.1007697. Epub 2015 Feb 10. PMID: 25668438.
- Hanson A, Haddad LM. Nursing Rights of Medication Administration. [Updated 2023 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560654/.
- https://www.drugsafetymatterspod.org/618871/14599616 (20.03.2024)
- https://urpl.gov.pl/pl/produkty-lecznicze/monitorowanie-bezpiecze%C5%84stwa-lek%C3%B3w (20.03.2024)
- https://medicalguidelines.msf.org/en/viewport/EssDr/english/drug-quality-and-storage-16688167.html (20.03.2024)
- Shafaat, K., Hussain, A., Kumar, B., Hasan, R.U., & Yadav, V.K. AN OVERVIEW: STORAGE OF PHARMACEUTICAL PRODUCTS. WJPPS. 2013; 22, (55): 2499-2515.


