The Market Access (MA) topic is fascinating in itself, but like any topic, it will be more easily remembered and assimilated if it is described in a non-standard way. Instead of using official definitions regarding Market Access, let’s go through the subject of MA on the example of Advanced Therapy Medicinal Products (ATMPs). Quite challenging? Probably yes, but like they say: where there’s a will, there’s a way.
Market Access „4 x right” definition
Market access definition may be described in one sentence as getting the right treatment to the right patient at the right time, and possibly even at the right price.
For the manufacturers and Marketing Authorisation Holders (MAHs), the ideal outcome of MA is to achieve the optimal price with maximum reimbursement rate for the approved target population with no limitation on prescription or pounding procedures. However , in practice there is a trade-off between:
- Price and reimbursement (P&R) conditions;
- Target population selection;
- Prescription and founding procedures.
The concept of MA was the ability to access market by pharmaceutical product without obstacles such as: obtaining a Marketing Authorisation Approval (MAA) the P&R levels, logistic (storage and supply conditions), the drug surveillance (follow up on potential and actual product Adverse Effect) etc. Nevertheless, in practice, the pharmaceutical industry has become capable of addressing all these hurdles except P&R. Thus, for industry the MA has become synonymous to the hurdle of achieving high P&R levels [1].
The other process that will appear in the topic of market access is the Health Technology Assessment (HTA). Once a medicine is approved for marketing, HTA bodies (in Poland: Agency for Health Technology Assessment and Tariff System; AOTMiT) are responsible for assessing its real-life efficacy (i.e. effectiveness), cost-effectiveness, relative efficacy, related medicinal need, budget impact and other evidence that will be later used by payers for P&R decisions, as well as for formulary listing criteria and prescription guidelines. Mysterious term ‘Formularies’ means the lists of medicines that may be prescribed at the ex

The question is who is the payer?
Payers are generally entities that finance or reimburse the cost of health services. In the healthcare market, payers act as gatekeepers for MA.
In most EU countries, there is one main payer in each country, corresponding to national public health insurance (in Poland: National Health Found, NFZ). Sometimes there are additional payers at regional level (e.g. in Italy), or a mix of national and fragmented private payers like in United States (US) [1].
Table 1 shows examples on how the patient access may differ between selected EU countries [2].
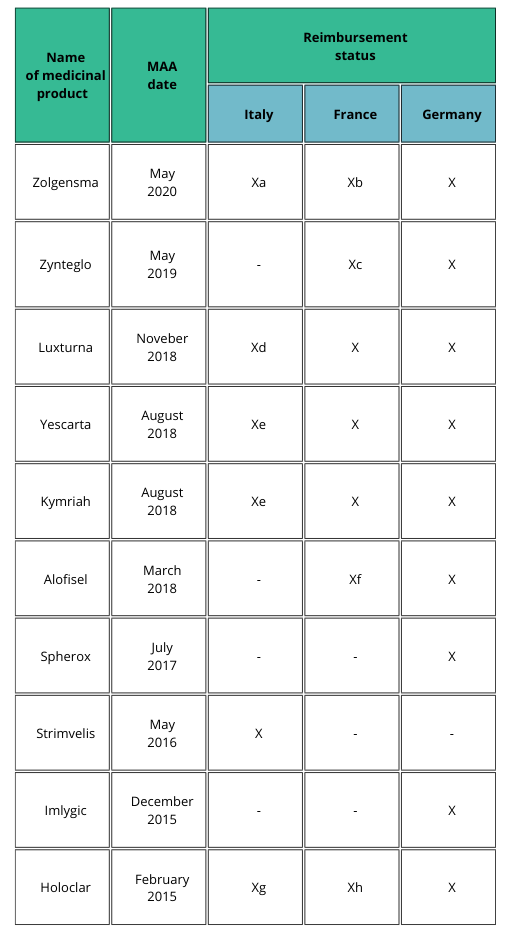
a) Patients weighing up to 13.5 kg and clinical diagnosis of SMA type 1 and onset of symptoms during the first six months of life, or genetic diagnosis of SMA type 1 (biallelic mutation in SMN1 gene and up to two copies of the SMN2 gene); AIFA registry mandatory to select eligible patients and to monitor treatment response, even for the management of risk-sharing agreement (payment at result).
b) Recommendation for reimbursement in the treatment of patients with spinal muscular atrophy 5q (biallelic mutation of the SMN1 gene), with a clinical diagnosis of type I and II SMA or presymptomatic and having up to three copies of the SMN2 gene.
c) Favorable opinion for reimbursement only in patients over 12 years to less than 35 years in the treatment of transfusion-dependent β-thalassemia (TDT), without β0/β0 genotype, eligible for hematopoietic stem cell transplantation (HSC), but not having related donor HLA compatible.
d) AIFA registry mandatory to select eligible patients and to monitor treatment response.
e) AIFA registry mandatory to select eligible patients and to monitor treatment response, even for the management of risk-sharing agreement (payment at result).
f) In the sole treatment of uncomplicated complex perianal fistulas in adults with nonactive/ slightly active luminal Crohn’s disease.
g) AIFA registry mandatory to select eligible patients and to monitor treatment response, even for the management of risk-sharing agreement (payment by results).
h) In the treatment of patients with moderate to severe limbal stem cell deficiencies, caused by chemical or physical eye burns, who meet the following criteria: presence of a superficial corneal neovascularization in at least two quadrants of the cornea in at least one of the eyes and involvement of the central cornea and severely altered visual acuity.
ATMP current status in EU
Obligatory regulatory pathway for ATMPs is the centralized authorisation procedure carried out by EMA. Once granted by the EU Commission, the centralized marketing authorization is valid in all member states. It could therefore seem that since the EMA assessed the product as safe, of adequate quality and efficacy and the authorization is valid in all countries, patients should have the same consistent access to the medicine. In practice, it turns out that nothing could be more wrong.
Despite the unification of the procedures for drug approval, each country is responsible for an effective national market access, which needs the P&R decision to be adopted. This can result in patient access inequalities among European countries, due to differences in terms of willingness to pay and opinion about the therapeutic value assessed by HTA bodies [2].
Let’s go deeper into ATMPS Market Access
Having been familiarized with some basics let’s try to define TOP challenges that ATMPs overcome during getting into the EU market. The list will be focused on challenges concerning strictly Market Access issues, apart from the challenges that arise during the Marketing Authorisation process.

- Limited efficacy and effectiveness data – lack of demonstration of efficacy and effectiveness for ATMPs has been identified by both regulatory agencies and payers. For three out of four EMA-rejected gene therapies and two out of three approved ones, there was a limited evidence demonstrating efficacy. This seems to be huge concern from the MA point of view since payers are not willing to pay for uncertain benefits. It was reported that 100% of Imlyfic, Luxturna, Glybera HTAs, and 50% of Strimvelis, Yescarta and Kymriah HTAs noted uncertainty regarding efficacy.
- Uncertainty in long-term benefits – designing clinical trial (CT) for ATMP is very challenging and non-standard. What distinguishes ATMP’s CTs from ordinary medicinal products is, among others: CTs for ATMPs are performed on small clinical trial sizes and they are mostly short-term CTs. Although long-term benefit can be extrapolated from these trials, but limited knowledge on appropriate methods to do so results in uncertainty in long-term benefits. This uncertainty influences the value perception by payers because they are concerned that retreatment might be needed in the future. You may ask – what is the problem with the retreatment procedure? The thing is, the retreatment comes with unknown safety and efficacy, as well as considerable additional costs which are not welcomed by payers. From interesting facts: 100% of Luxturna, Glybera, Yescarta, and Kymriah, and 50%of Imlygic HTAs noted uncertainty regarding duration of effects.
- Lack of appropriate comparator – Finding an appropriate comparator for ATMPs can be difficult, especially when the therapy can lead to practical changes in clinical practice (e.g., need for additional services and facilities) or in case there are no existing treatments, which is quite common in case of ATMPs. On what scale is this a problem? Here is the answer: 100% of Imlygic, Yescarta and Kymriah, and 50% of Strimvelis HTAs noted a lack of appropriate comparative data.
- High short-term costs – ATMPs in general are developed to only require one or a limited number of administrations – you may say that this is an advantage of ATMPs. From the patient’s point of view, who does not need to undergo long-term treatment – for sure it is advantage. But for payers it means high short-term costs. Examples include 1.1 million EUR for Glybera, which was only used for one patient in Germany [6]. These high prices perceived by some as an affordability threat for healthcare systems. Although one ATMP is unlikely to endanger the sustainability of healthcare budgets, it remains to be seen whether the cumulation of ATMPs could become problematic for payers. Sustainability problems could become more prominent when ATMPs for common diseases make their way to the market, although this might also provide opportunities to decrease prices. Nevertheless, it should be highlighted that cost of ATMP manufacturing (in GMP requirements) is also high therefore, there is no expectation that ATMP drugs will be cheap [3]. The question is therefore, whether health systems are ready for the presence of modern drugs such as ATMP on a larger scale? What do you think?
- Uncertain cost-effectiveness – Effectiveness and, therefore, also cost-effectiveness, are uncertain at the time payers need to make a decision because: (i) ATMPs often receive MA based on limited clinical evidence (conditional MA) and (ii) lack of comparative effectiveness data. Again, an analysis of ATMP HTAs identified that 100% of Luxturna, Yescarta, and Kymriah, as well as 50% of Imlygic HTAs noted uncertainty around cost-effectiveness.
- Uncertain direct and indirect costs – ATMP therapeutic costs are one thing and do not include the ancillary medical costs of administering them, treating complications, and follow-up care. Evidence can be lacking for these costs in the intended-to-treat population. Apart from healthcare cost, there are also costs that patients and their families might incur because they are required to travel to administration sites, often close to a manufacturing site, and to stay there for follow-up monitoring. These costs are also quite high.
- Heterogeneity in requirements – Besides regulatory procedures, procedures to obtain reimbursement are also demanding and differ between EU countries and sometimes even between regions within one country. HTA requirements and methodologies also vary across Europe leading to differences in access for patients. Moreover, evidence requirements of regulators are often very different from those of HTA bodies and payers (e.g., regulators’ acceptance of limited clinical evidence is higher than that of payers), making it difficult to be aware of, and navigate among, all requirements.
- Issues in valuation of benefits – ATMPs are challenging HTA practices because: (i) unique attributes that influence the value proposition are not acknowledged; (ii) evidence regarding unique promising technology attributes (e.g., long-term benefits) is not available or uncertain; and (iii) there is no clear methodological approach for these unique value scenarios, making it difficult to generate the robust health economic estimates that form the basis of any value assessment. For example, in research commissioned by NICE in 2017, it is reported that current NICE methods were likely to appropriately assess uncertainties of ATMPs, but that standard assessments might not be sufficient [7].
- Assessor’s experience and preparedness – due to the fact that ATMP products are not so common assessors do not have many opportunities to gain experience in documentation evaluation. Lack of appropriate tools and experience can lead to delayed assessments and uncertainty over how these products will be assessed and reimbursed.
- Manufacturers awareness – not only assessors (HTA bodies) are not experienced in ATMP reimbursement policy but those who develop ATMP neither. The fact that ATMP development starts in many cases in universities or other scientific entities makes the gaining reimbursement more challenging since the actual steps of having conversations with stakeholders are neglected due to lack of awareness of scientists on the subject of reimbursement regulations.

Short summary
There is no denying that in the context of market access for ATMPs, there are many challenges – not only those described above, but also related to the MAA gaining (central MAA), the manufacture of this product (up-scaling issue,) and the coordination of the entire product development process (covering CT designing). It is however worth remembering that all challenges can be transformed into opportunities – and this may also be the case here. The factor that will help make this change is certainly the increase in interactions between the various entities involved: HTAs, developers/manufacturers, payers, regulators and of course: patients.
Do you have any questions about Market Access of ATMPs? Remember that SciencePharma is here ready to help in case of ATMP issues. Contact us directly, our experts will be happy to provide you more details.
References:
[1] Güvenç Koçkaya, Albert Wertheimer; Pharmaceutical Market Access in Developed Markets, January 2018.
[2] Lucia Gozzo, Giovanni Luca Romano, Francesca Romano, Serena Brancati, Laura Longo, Daniela Cristina Vitale, and Filippo Drago; Front. Pharmacol., 08 October 2021.; Health Technology Assessment of Advanced Therapy Medicinal Products: Comparison Among 3 European Countries.
[3] Eline van Overbeeke, Sissel Michelsen, Mondher Toumi, Hilde Stevens, Mark Trusheim, Isabelle Huys and Steven Simoens, Drug Discovery Today Volume 26, Number 2, February 2021; Market access of gene therapies across Europe, USA, and Canada: challenges, trends, and solutions.
[4] EMA Annual Report 2021; The European Medicines Agency’s contribution to science, medicines and health in 2021.
[5] EMA Annual Report 2020; The European Medicines Agency’s contribution to science, medicines and health in 2020.
[6] Seppo Ylä-Herttuala; Glybera’s Second Act: The Curtain Rises on the High Cost of Therapy; www.moleculartherapy.org vol. 23 no. 2 february 2015.
[7] Hettle, R. et al. (2017) The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol. Assess. 21, 1–204.

