
-
Product Development
-
Clinical Trials
-
Quality
-
GxP Audits
-
Regulatory Affairs
-
Pre-authorisation
- Regulatory Roadmap
- Scientific Advice
-
Product information
-
Dossier preparation
- Dossier Gap Analysis
- MA Handling
- Post-authorisation
- Regulatory outsourcing
-
Pre-authorisation
-
Pharmacovigilance
-
QP Service / Importer
Periodic Safety Update Report (PSUR)
A Periodic Safety Update Report (PSUR) is a regularly prepared document aimed at monitoring and analyzing the safety of a medicinal product throughout the entire duration of its marketing authorization (MA). The PSUR is prepared on the basis of new or emerging safety information, including those from clinical and non-clinical studies, reviews of scientific literature, descriptions of adverse reactions, and sales information available from the Marketing Authorization Holders (MAHs). This document is designed to evaluate the risk-benefit balance of a medicinal product at defined time points during its post-authorisation phase.
Would you like to learn more about the PSUR?Would you like to learn more about the PSUR? Check out our blog: Periodic safety update report (PSUR) – essential facts that you should know
What are the challenges of preparing a PSUR report?
The legal requirements for the submission procedure of PSURs are established in Regulation (EC) No. 726/2004, Directive 2001/83/EC, and the Pharmaceutical Law of 6 September 2001. The format of PSURs shall follow the structure described in Implementing Regulation (EU) No. 520/2012, while the scope, objectives, and content of the PSUR are described in the guidelines of Module VII of Good Pharmacovigilance Practice (GVP). Due to the broad legal framework and continuously updated guidelines, the MAH must adopt an interdisciplinary approach to the PSUR preparation process. Therefore, cooperation between experts in the fields of medicine, science, law, and regulations is essential. Only this combination of expertise allows for the proper preparation of a high-quality document that does not raise concerns from the Competent Authorities (CAs).
The deadlines for submitting the report are strictly defined by the European Medicines Agency (EMA) and presented in the monthly updated List of European Union Reference Dates, the so-called EURD List. It is an extensive list of active substances and combinations of active substances contained in medicinal products subject to safety assessment at the European Union level.
The EURD list is a legally binding document that should be constantly monitored by each MAH in terms of:
- Frequency of PSUR submission (i.e., 6-monthly, yearly, 3-yearly).
- Data lock point (DLP): This is the cut-off date for data to be included in a PSUR.
- PSUR submission date: Once the DLP is reached, reports should be submitted within 70 days (for reports covering up to 12 months) or 90 days (for reports covering more than 12 months).
- Requirements for the submission of PSURs for generic, well-established use, homeopathic, and traditional herbal products.
In SciencePharma, we regularly check the EURD list updates under the Regulatory Intelligence process.
The list does not include substances assessed at the national level. For these active substances, the frequency of submission is established at the national level. In such cases, the submission deadline is specified by the National Competent Authority (NCA) in the MA.
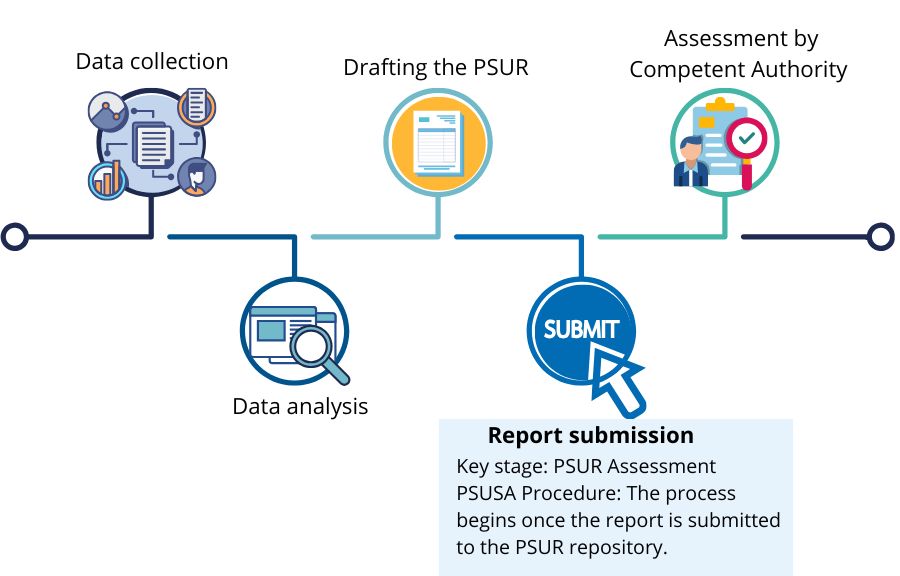
Key stage of the procedure – PSUR Assessment
Each MAH whose medicinal product requires the submission of a PSUR is legally obligated to submit the report directly to the PSUR repository. This initiates an important stage of the assessment process known as the EU PSUR Single Assessment Procedure (PSUSA). The PSUSA procedure allows to assess PSURs of medicines containing the same active substances or combinations, even if they are subject to different marketing authorisations and are authorised in different EU Member States. This aims to harmonise and strengthen the benefit-risk review of medicines across the European Economic Area. As part of this procedure, the Pharmacovigilance Risk Assessment Committee (PRAC) assessing the PSUR may request additional actions from the MAH, such as submitting a response or providing additional information to the report, to ensure that the safety of the product is adequately monitored.
Why should you start cooperating with SciencePharma?
SciencePharma provides pharmacovigilance support covering the entire life cycle of a medicinal product. We tailored our business offer to the needs of our clients in a flexible and personalized way. We offer the possibility of preparing a full PSUR report or providing the MAHs with detailed safety information that is necessary for further actions and decision-making. The process of preparing the PSUR by qualified SciencePharma experts has been repeatedly verified during inspections by the CAs, including URPL (Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products) and EMA. Moreover, we have experience preparing PSUR reports for non-EU countries, such as Indonesia, Ukraine, Belarus, and Canada. Therefore, cooperation with us is a guarantee that all reports will be prepared in accordance with legal requirements, within specified deadlines, and at the highest level of quality.
Thanks to an individual approach and an advanced drug safety management system, we help clients focus on the development of their products.

