
Import and supply chain management
For all the Marketing Authorisation Holders (MAHs) of medicinal products manufactured outside the EU/EEA territory, that wish to introduce it into EU market, there is a need to have the medicinal product importer responsible for batch certification. Medicinal products import to EU countries is one of the core services provided by SciencePharma in terms of supporting the presence of medical products from third countries (outside the EU / EEA) on the European market. We provide importation services comprehensively and ensure professional verification of compliance with EU legal regulations and GMP/GDP.
The EU GMP requirements for importer regarding medicinal products manufactured outside the EU
The requirements applicable when importing medicinal products to the EU are described in the EU GMP guideline and summarized in new Annex 21 to the EU GMP, which came into force in August 2022. Following the Annex 21, within the importation process, we can distinguish the following places linked to their corresponding activities:
- the site of physical importation with the primary role of receipting the imported product,
- the site of batch certification or – for bulk or intermediate products that are further processed – QP confirmation.

The EU importer – MIA Licence and GMP-compliant pharmaceutical quality system
Given their specific importation responsibilities the above-listed sites are required to hold relevant Manufacturing & Import Authorisation Licence (MIA Licence) and appropriate GMP-compliant pharmaceutical quality system implemented considering the scope of the activities carried out in place. At SciencePharma we fulfil this requirement and proclaim as a qualified partner employing highly experienced specialists who can comprehensively carry out the import process. SciencePharma is a holder of its own MIA licence. If needed, we can also rely on the MIA of our proven, long-term partners providing one-stop-shop importation services for all importation activities needed.
SciencePharma provides the following importation services: organizing transport worldwide, storage, distribution import and supply chain management.
SciencePharma’s experts have wide experience in managing medicinal products importation. We work in accordance with the Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP). We provide importation services extensively, taking care of all the stages including: worldwide transport organisation, storage, distribution and supply chain management. A substantial part of our activity is batch control studies in contract quality laboratories located all over the EU.
We do have extensive knowledge and experience in the field of:
- transportation (air, road, sea and rail transport) in partnership with reliable shipping companies,
- custom clearance in cooperation with Custom Agencies (in manufacturing and destination countries)
- storage conditions assessment
- product storage at the site of physical importation
- complaints and quality defects management
- serialization alerts supervision
- commissioning and supervising of quality control studies
- management of analytical methods transfers to laboratories located in the EU
- warehouse organisation and management
- comprehensive supply chain supervision and optimization in terms of time and cost savings
… and many other activities, which must be performed when launching a drug in the EU market. The evident and substantial part of our import activity is performing batch analysis in contract Quality Control Laboratories located all over the EU. For completing the comprehensive service, we also provide the highest quality QP services (batch certification) and batch release.
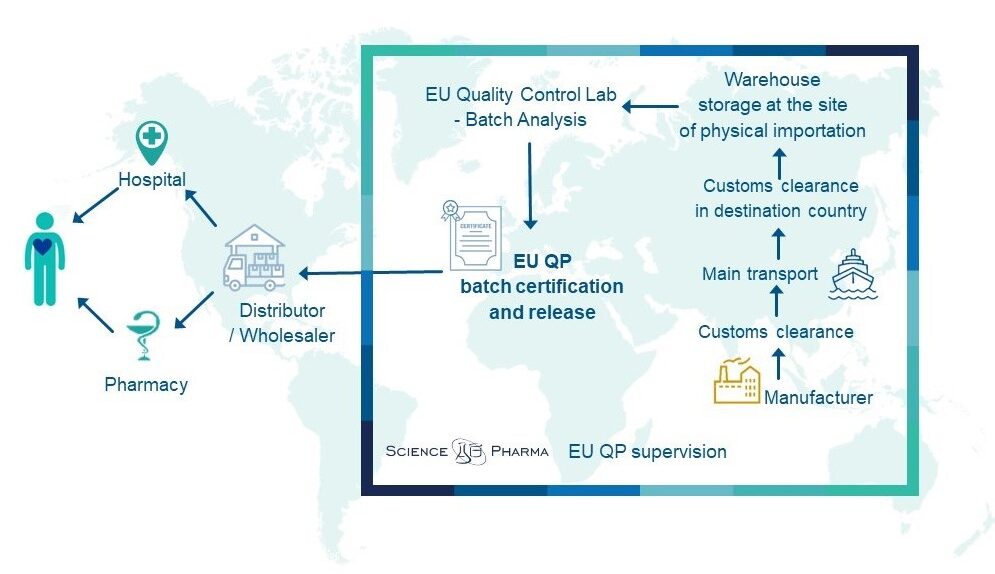
Distribution process
What’s more, we supervise the distribution process in accordance with GDP, starting from the point of product order, throughout the importation process and storage. We control all the logistic issues and ensure the quality of the distributed products is maintained at the level that meets the GDP requirements during the entire distribution process.
Additionally, our specialists have experience in sourcing from the market and transportation reference products needed for developing CMC studies and for clinical trials – both from the EU and third countries. In the case of investigational medicinal products we organize IMPs distribution for clinical studies, assuring IMPs delivery to individual clinical sites under appropriately controlled conditions, in line with existing legal regulations and GMP/GDP recommendations. We also offer investigational medicinal products for clinical studies re-packaging coordination in compliance with GMP.
SciencePharma experts have experience in providing manufacturing and importation services worldwide
SciencePharma provides services to small, medium and large pharmaceutical companies located worldwide. Our experts have experience in manufacturing and importation activities, serving Clients from third countries manufacturing their medicinal products in Asia and South America as well as in the US, and in European countries beyond the EU territory. Our advantage comes from in-depth knowledge of EU law and national specific legal requirements, procedures and expectations of the registration agencies in EU countries, which is the key for success in both importation of products and the overall pharmaceutical regulatory support and projects management.
Planning a medicinal product import?
We are here to help you with all of the regulatory, transportation and custom clearance challenges. Cooperation with us gives you guarantee of smooth and trouble-free import. Let us deal with all the handling and import formalities on your behalf.

