
Nitrosamines – risk analysis
In June 2018 EMA started to recognize that some active substances contain undesired presence of carcinogenic nitrosamine (NA) impurities [e.g. N-Nitrosodimethylamine (NDMA), N-Nitrosodiethylamine (NDEA)] and started to develop ways of minimizing that risk. Our article discussing this problem is available here.

There have been reports of withdrawing batches of various medicinal products containing undesired levels of NA. As a result, EMA, CMDh together with national Agencies requested all Marketing Authorization Holders (MAHs) to review manufacturing process for products containing chemically synthesized or biologically active substances and perform respective risk analysis (RA) for the presence of NA impurities in their products. The RA outcome should be then presented to the competent authorities. Furthermore, if the presence of NA could be of a concern, further steps are required, such as confirmatory testing or even subsequent changes in the active substance and/or drug product manufacturing process including stricter controls. The latter would also require a subsequent variation(s) to Marketing Authorization.
Risk analysis step by step
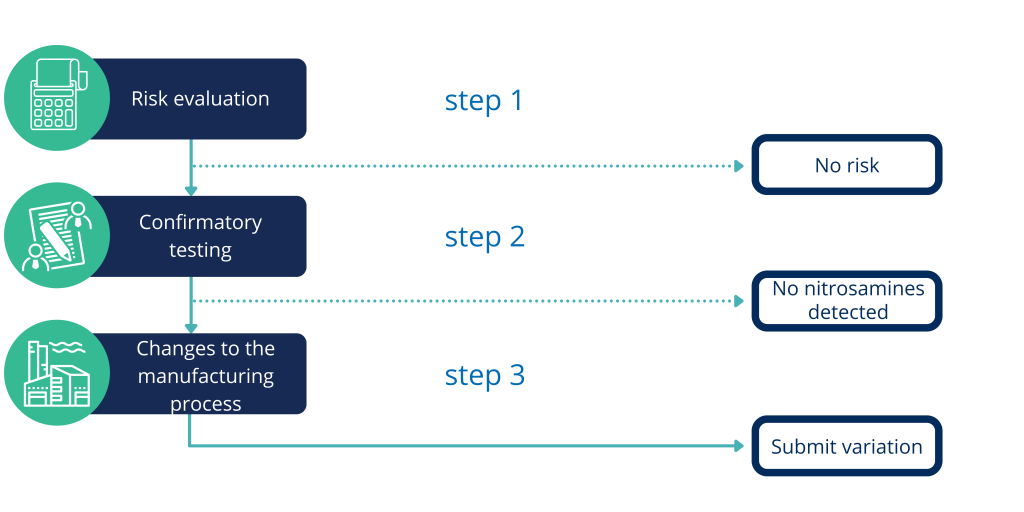
We can guide you through that process, in which we can help you with assessing the above discussed risk. We can perform full risk analysis, as well as assess the existing ones.
Who we help?
The following cases are not exclusive list of cases in which we are able to help:
- MAH has not performed RA yet and doesn’t have the knowledge of how to do it.
- MAH performed RA, but doesn’t know how to proceed, where to include the analysis, whom to contact.
- The API manufacturer considers certain steps in chemical synthesis, but is not sure what would be the impact on the NA formation.
- Product manufacturer wants to assess whether its current or new product is under the risk or NA formation.
Our services are dedicated to everyone who needs to perform RA for NA, that includes: MAHs, Pharma companies, Drug Product manufacturers or API suppliers
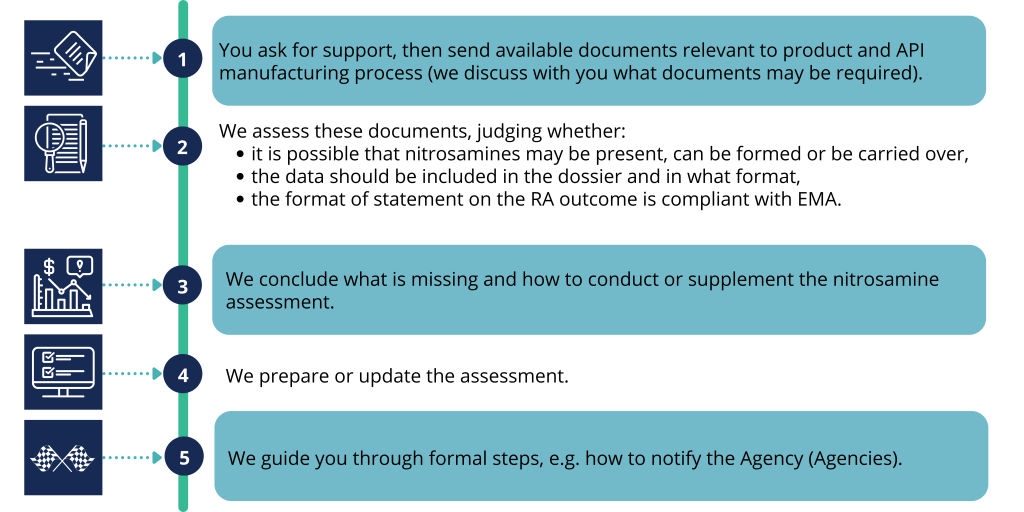
Stages of cooperation
An example of cooperation may consists of the following:
Do you know that…
As observed, NA problem can lead to delays during registration process or even product batches being withdrawn from the market. Hence, appropriate actions should be ideally made as early as possible to avoid such unexpected events, quality assurance issues and holding times during product supply.
Cooperation with us will help you go through the NA assessment process and decrease the discussed risks.
Why is it worth it?
SciencePharma has broad range of experts on board who can assess the risk of nitrosamine formation from various angles (scientifically, regulatory). We find our collective knowledge and experience important to help assess whether there is any risk of nitrosamine impurities in the active substance or product. We also help in proceeding with respective outcomes in the EU Agencies on national and international level (DCP, MRP, central). Altogether, our services may be very helpful if you find yourself in the need to have such analysis performed or reviewed.

At the end
Nitrosamine impurities have recently become one of a key quality aspect during active substance and drug product manufacturing. Respective risk assessments are now obligatory for MAHs and their outcome is of key importance for the potential process design.
If you want to sustain a flawless business, have a risk of nitrosamine impurities assessed with appropriate Agencies informed, SciencePharma can help you go through it.

