
Paediatric Investigation Plan
What is a Paediatric Investigation Plan (PIP)?
The paediatric population is a unique group of patients requiring tailored pharmacological treatment. According to Regulation (EC) No 1901/2006, a Paediatric Investigation Plan (PIP) is a detailed research and development program of medicinal product to be authorized for treatment of paediatric population. This document is required by the European Medicines Agency (EMA). It ensures that medicinal products used to treat children undergo high-quality, ethical studies and are authorized for the use in the paediatric population.
For which medicinal product applications is the PIP prepared?
Under Regulation (EC) No 1901/2006, each application for marketing authorization for a new medicinal product must include:
-
the results of all studies performed and details of all information collected comply an agreed PIP, including the decision of the Agency approving the PIP, or
-
the Agency’s decision to grant PIP-waiver (for a specific product or for a specific class, if they meet the criteria), or
-
the Agency’s decision to grant a deferral of the beginning or completion of some or all of the activities included in the PIP.
This requirement applies to all original, fixed combination, and medicinal products registered as a result of the informed consent. It also applies to authorized medicinal products protected by a supplementary protection certificate or patent, in case of registraion of new indication, new pharmaceutical form or new route of administration.
What does PIP include?
In relation to a given indication, a PIP covers various aspects of using a medicinal product for children:
-
differences and similarities between adult and paediatric populations,
-
current methods of diagnosis, prevention or treatment in paediatric populations,
-
description of the fulfilment of therapeutic needs and/or significant therapeutic benefits,
-
overview of the available data about the medicinal product,
-
proposed strategy for the development of the medicinal product, including descriptions of non-clinical, clinical or modelling/simulation studies.
After approving the PIP, it is continually updated using modification procedures.
Understanding PIP-waiver
The requirement to submit PIP may be waived in certain situations. This applies to specific medicines or even classes of medicines that:
-
are likely to be ineffective or unsafe for part or all of the paediatric population, or
-
are intended for conditions occurring only in adults,
-
offer no significant therapeutic benefit over existing treatments for paediatric patients.
As mentioned above, there are generally two types of waivers:
- product-specific waivers,
- class waivers.
An appropriate and detailed justification is required to issue a waiver for some or all paediatric subsets, either in relation to one or more specific therapeutic indications, or in relation to both. The Paediatric Committee adopts class waivers on its own motion.
PIP application assessment procedure
The applicant submits the Paediatric Investigation Plan to the Agency along with a request for agreement. Within 30 days of receiving the application, the Agency shall verify the validity of the application and prepare a summary report for the Paediatric Committee. If necessary, the Agency may request the applicant to complete data or submit additional documents, which will result in the suspension of the 30-day deadline until providing the required supplementary information. After completion of the validation stage, the Paediatric Committee evaluates PIP application. Assessing the PIP application takes the form of max. 120-day procedure.
Within 60 days, the Paediatric Committee shall issue an opinion stating whether the proposed research will provide the necessary data. Both the applicant and the Paediatric Committee have the option to request a meeting during this period, and/or the applicant may receive an invitation to propose modifications to the plan. In the event of a request to modify the plan on the 60th day, the deadline is suspended until providing the requested information. On the day 61, after receiving the applicant’s response, the procedure starts again and runs until day 120, when the Paediatric Committee issues the opinion (positive or negative).
If, after considering the Paediatric Investigation Plan, the Paediatric Committee concludes that the requirement to provide additional information is waived, it may adopt an opinion in favor of a waiver. An application for PIP-waiver may also be submitted by the applicant, and the procedure is the same as in the case of the PIP assessment.
Once the Paediatric Committee has issued its opinion, the Agency has 10 days to transmit it to the applicant. Within 30 days the applicant has the opportunity to ask the Agency to re-examine the opinion (with detailed justification). If the applicant does not request a re-examination, the opinion of the Paediatric Committee shall become final. Agency shall adopt a decision not later than 10 days from the date of receipt of the final opinion of the Paediatric Committee. This decision shall be communicated to the applicant in writing.
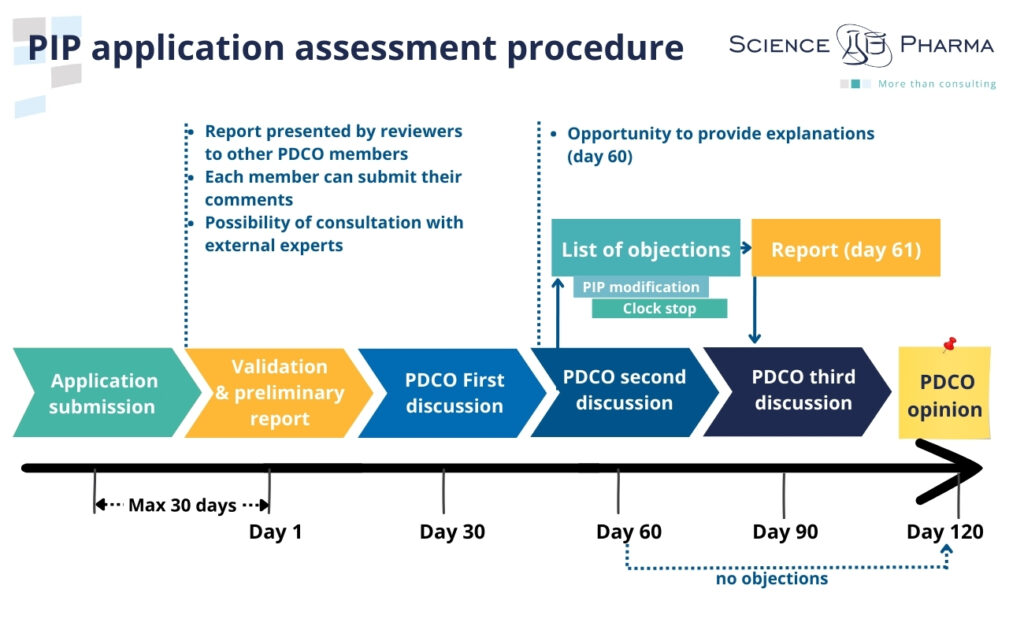
If, after obtaining the decision approving the PIP, the applicant encounters difficulties in carrying out clinical trials on the paediatric population, they may propose modifications or submit an waiver or deferral application to the Paediatric Committee, providing appropriate justification. The Paediatric Committee examines the modifications or application and issues an opinion within 60 days.
Incentives for Compliance – 6-month extension of a supplementary protection certificate
To motivate pharmaceutical companies to conduct research on the paediatric population, the EMA offers a six-month extension of protection period resulting from the granted patent orsupplementary protection certificate (SPC). This extension of protection is possible for medicinal products covered by the requirement to submit paediatric data. This reward applies to the medicinal product after getting marketing authorization (MA) in all European Union Member States. Another reqirement to obtain this protecion for medicinal product is to conduct studies in accordance with the approved PIP and include their result in the summary of product characteristics (SmPC). This also applies to situations where the medicinal product is not approved for marketing for paediatric indications, but information on the results of clinical trials in children will be included in the SmPC.
In order to protect children’s health, a number of legal documents have been developed regulating the manner of presenting data in PIP (PIP-Templates and forms), as well as conducting clinical trials involving paediatric population. This information is collected in the form of guidelines, which, if followed, minimize the risk of questions from the Paediatric Committee.
Why Choose SciencePharma for the PIP preparation?
Our experience shows that even with carefully prepared documents, the Paediatric Committee of the European Medicines Agency may have a number of questions. At SciencePharma, we leverage our extensive knowledge and experience to anticipate these questions, creating high-quality documentation that the minimizes risk of Expert comments and helps adhere to project deadlines and budget.
At SciencePharma we offer:
-
Comprehensive analysis: We analyse available data on the medicinal product and determine the scope of non-clinical and/or clinical studies for paediatric population, if required;
-
PIP Preparation: We prepare PIPs that comply with the latest guidelines;
-
PIP Waivers: We assist preparation of documents required in obtaining PIP waivers, including class waivers, and support through the process.

Benefits of Partnering with SciencePharma
At SciencePharma, we offer tailored solutions, with customized approaches for each Client’s unique needs. We come at each project with passion and commitment, and our experience in research and registration of products from many therapeutic areas allows us to take an objective and comprehensive line to the analysed topic. We are constantly expanding our knowledge about current requirements and registration procedures by participating in the internal and external trainings, which translates into the quality of the documentation we prepare. Thanks to many discussions with the competent authorities, we can foresee the regulatory questions in advance and prepare comprehensive answers, reducing delays and extra costs.
If you are looking for a partner to assist you with your Paediatric Investigation Plan or PIP waiver, contact us today. Our team is here to support you at every stage of this process! You may also wish to explore our other clinical trial services check – Clinical Trials.

